What is the current Status of GDP Implementation?
The distribution of medicinal products is often complex, involving many different parties. In addition to the challenges associated with this complexity, there is also a growing threat from criminal activities seeking to introduce falsified medicines into the legal supply chain. Taking into account the changes in the global distribution, a revised version of the EU-GDP Guidelines was issued in 2013. The guidelines now have a stronger focus on the quality system with clear responsibilities and processes and the application of risk management principles. More detailed guidance has also been developed to prevent falsified medicines entering the legal supply chain.
On the fifth anniversary of the revised EU-GDP Guidelines, the European GDP Association (EGDPA) decided to evaluate the current implementation status of these guidelines within the pharma industry. The survey prepared by the EGDPA sought feedback from the manufacturers, wholesalers and the transportation companies. The response was overwhelming, almost 100 companies across Europe responded to the survey.
Almost half of the respondents were from pharmaceutical manufacturers and 30% from wholesalers, others mainly from distribution service providers and transport companies. All provided valuable insights in some of the most important fields of GDP. As the guidelines mainly apply to wholesalers and manufacturers, their answers have been evaluated separately from those of distributers and transporters.
Auditing
Only about 20% of manufacturers perform audits of the hubs at airports, whereas distributers seemed more active in this field; with 42% perform these audits. To limit the duration of storage of medicinal products at airport hubs, more than half of the respondents apply a risk assessment processes. Independent from that, 42% try to keep their products less than 24 hours at the hubs. Distributers try to be more efficient here and 75% tend to get products out as quickly as possible.
At least 85% of the manufacturers audit their third party logistic service providers and their contract transportation companies before approval and implementation. However about 14% do not audit transport companies at all or simply rely on the selected company's "good reputation".
Falsified Medicines
This is one key goal of the new guidelines: to prevent falsified medicines from entering into the legal supply chain. More than half of the respondents from manufacturers and wholesalers have at least some written procedures describing how to deal with falsified medicines (and about 90% of the respondents from distribution and transport - which sounds a bit surprising). Most companies train all their employees on how to act when there is a suspicion of falsified medicine. However, it seems that there is room for improvement when it comes to the question how to 'actively look for falsified medicines', only a third of the respondents said they actively monitor all their suppliers. Unfortunately 11% do nothing!
Implementation of the 2D Matrix Code
In February 2019, security features will be mandatory on the packaging of all prescription drugs, as well as those of critical over-the-counter drugs. These security features will allow for their identification and verification of the authenticity of the medicines supplied at the point of sale. The outer packaging or, if not available, the immediate packaging of the medicinal products supplied within EU must have safety features to allow for its verification and tampering. The verification will be implemented via a unique 2D matrix code on each pack that can be read by common scanners. The technical and organisational details regarding the security features were published together with the Delegated Regulation (EU) 2016/161 on 9 February 2016.. However, potential checks (and deactivations), also at wholesale and distribution level are being discussed depending on the way of distribution (e.g. products defects, returns, recalls etc.).
About 30% of the respondents from wholesalers and manufacturers thought that these requirements were not relevant to them. This also included companies operating outside the EU or manufacturers of Investigational Medicinal Products (IMPs), Active Pharmaceutical Ingredients (APIs), homeopathic products and radiopharmaceuticals. However, it is concerning that not all the stakeholders which need be involved in the implementation of the safety features (2D Matrix Code, and tamper evident feature) have started the implementation:
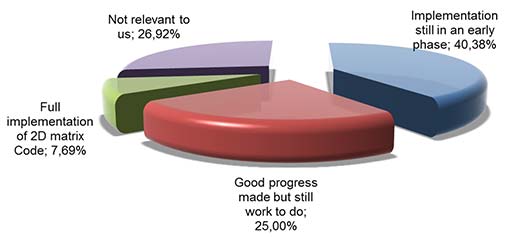
Picture 1: 2D Matrix Code - what is the current level of implementation? (wholesalers and manufacturers)
Temperature Control
The way, (product) temperature is monitored in the supply chain is quite heterogeneous: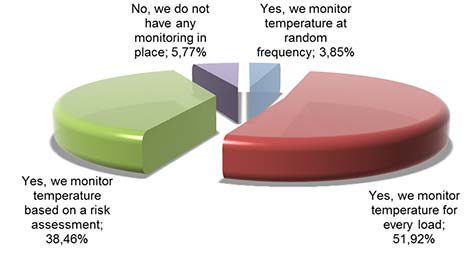
Picture 2: Do you monitor product temperature in your supply chain? (wholesalers and manufacturers)
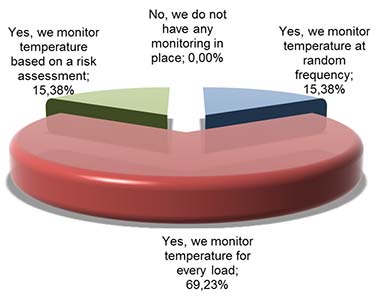
Picture 3: Do you monitor product temperature in your supply chain? (distributers and transporters)
But almost everybody is already mapping warehouses (94% of wholesalers and manufacturers, 100% of distributers and transporters) or is planning to do so (2% of wholesalers and manufacturers). 77% (resp. 92%) also have power back-ups if cold store rooms in case of power failure; the rest need will relocate their products if needed.
In summary the survey shows good progress within the industry towards full implementation of the EU GDP requirements, however, there are still some companies falling short of full compliance. The reasons for lack of compliance may be due to lack of resources, poor understanding of the risks associated with non-compliance, and lack of adequate supervision from the customers and regulators.
Companies should initiate a gap analysis of their current processes and procedures managing their operations against the requirements (as stated in the EU-GDP Guidelines). Once the gaps are identified, applying the principles of risk management should be prepared and actions assigned for implementation. Ongoing internal audits can identify further gaps and also assist in training and education of the staff.
The European GDP Association in collaboration with the Pharmaceutical Quality Group (PQG) have developed an implementation guideline for the EU-GDPs, practitioners are encouraged to download these guidelines (available free of charge) and use the practical solution provided in this document. The dcoument is available in the members' area on the GDP Association website. If you are not a member yet, simply apply for membership - it is free of charge. We remain hopeful that the pharmaceutical industry will successfully implement the EU GDP requirements which will reduce risk of poor product reaching the customers while increasing patient confidence on the medicines they receive.
The results of the survey can also be downloaded in the European GDP Association website members' area "Downloads" section.
Conference Recommendations
Related GMP News
GMP Conferences by Topics
- General Quality Assurance and GMP Compliance Topics
- Hygiene
- General Microbiology Topics
- Regulatory Affairs
- Development
- General Analytics Topics
- Good Distribution Practice
- Sterile Manufacturing
- Computer Validation
- General Qualification/Validation Topics
- General Engineering Topics
- APIs/Excipients
- GMP Basic Training Courses
- Medical Devices and Combination Products
- Packaging and Packaging Material
- Data Integrity
- Qualified Person (QP)
- GMP Auditing
- Documentation
- Cleaning Validation
- General IT Compliance Topics
- Impurities
- OOS / OOE / OOT
- Material Testing
- Validation of Analytical Methods
- Analytical Instrument Qualification
- Stability Testing
- Microbiological Testing
- Technology
- General Manufacturing Topics
- Solid Dosage Forms/Semi-Solid Dosage Forms
- Biotechnology/Blood/ATMP
- Herbal Drug Products/Cannabis/Radiopharmaceuticals
- Others

